Exactech Knee Lawsuit Attorneys | Knee replacement with an Optetrak, Optetrek Logic or Truliant Knee? Call 954-384-6114 – Exactech Lawsuit Lawyers
Exactech is Knee-Deep in Lawsuits
Exactech, founded in 1988, is a Gainesville-based medical technology company that specializes in the production of devices for use in joint replacement procedures. Exactech is currently under scrutiny over claims that their knee and ankle replacement devices are wearing out faster than expected. The shorter lifespan of these devices has forced users to undergo avoidable revision surgeries at its best, and health-threatening complications at its worst.
An Invisible Problem
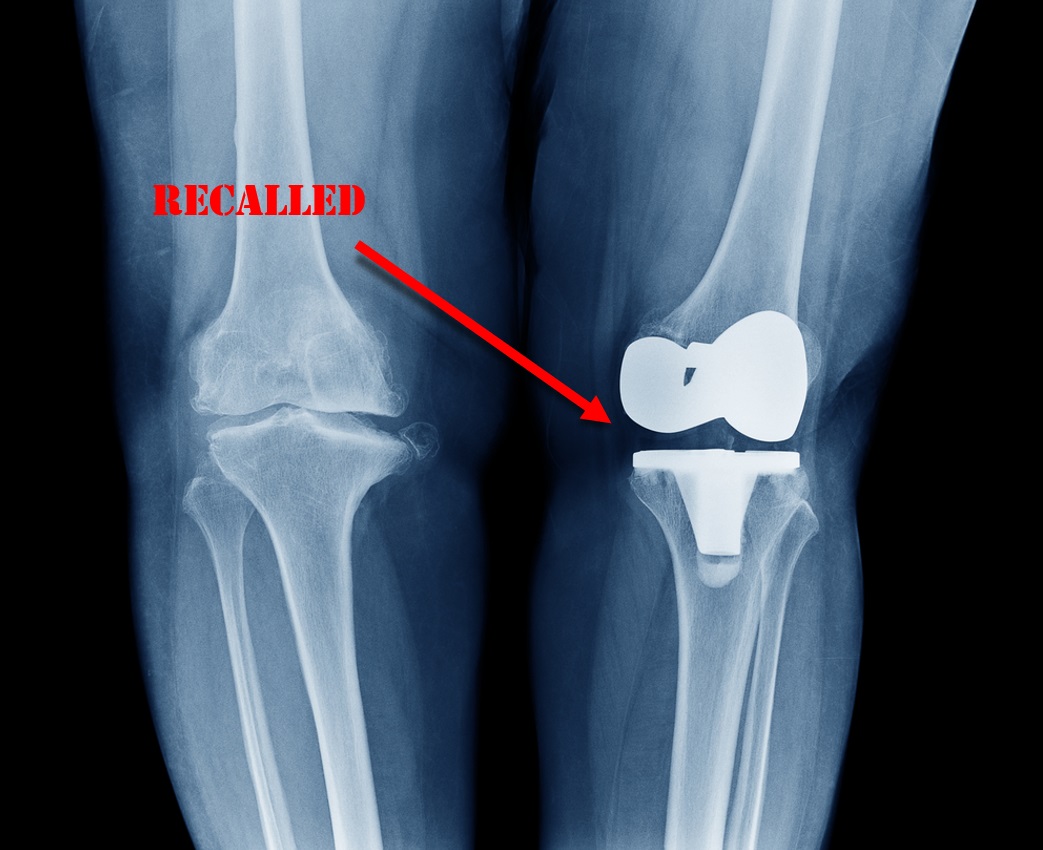
Exactech Knee replacement devices, specifically, the Optetrak, Optetrak Logic, Truliant, and Vantage Systems have prompted the company as well as the FDA to issue warnings and an Exactech recall. The Optetrak Logic system, for instance, marketed to have “excellent long-term implant survivorship” is an insert that is attached to both the femur and the tibia (the two bones that meet at the knee) to essentially act as an anchor point between the two bones. The device’s unique selling point is that it is designed to both maximize bone preservation and reduce wear.
In order for the Optetrak Logic System and the other devices to achieve both of those goals, the devices use a special technology that Exactech calls “Net Compression Molded Polyethylene” (NCPM), which is a combination of two design feats: the use of a special type of plastic called polyethylene and the compression of this material using a technique that is meant to reduce oxidation. Ironically, despite Exactech’s innovation in their product design attempting to mitigate oxidation, it seems the packaging of the product was not sufficient to guarantee the functionality of the product.
Picture courtesy business newswrire
Optetrak Logic Lawsuit
The problem Exactech faces arises because the materials used in knee and ankle replacement devices are all prone to oxidation, even their innovative NCPM, and this process is accelerated when the NCPM is exposed to oxygen from the air. Exactech allegedly knew this, and to address this problem they shipped their devices in vacuum sealed bags. However, just last year, Exactech realized that devices sold as far back as 2004 were shipped with defective vacuum-seal bags, which meant the devices were exposed to oxygen all the way up to insertion and thus degraded significantly faster than what was originally planned.
Picture courtesy PR Newswire
Truliant Knee Exactech Lawsuit
Early oxidation weakens the polyethylene plastic faster, resulting in the implants having malfunctions much earlier than expected thereby forcing users to undergo revision surgeries. To make matters worse, before users realize they need a revision surgery they may experience joint pain, swelling, difficulty walking, knee instability, grinding, clicking or other noises from the implant, and bone loss (osteolysis).
A Growing Recall
In August of 2021, Exactech disclosed problems with the Exactech replacement inserts. However many consumers did not know about the Exactech replacement inserts issue until February 7, 2002 when Exactech announced a world-wide recall of their Optetrak products containing polyethylene. The FDA classified this recall as a Class II recall, which means the product in question has the chance of causing “temporary or medically reversible adverse health consequences.” Later, on February 7, 2022, Exactech communicated that actually most of their inserts manufactured since 2004 were packaged in defective bags that do not isolate oxygen adequately, which led them to increase the magnitude of the recall. The most recent recall now includes three more types of inserts, including both knee and ankle replacement devices, and more than doubles the number of recalled units. Exactech sent their recall notice to orthopedic surgeons throughout the United States providing information as to the reason for the recall, and notifying the surgeons to provide the recall letter to their patients who had the defective devices.
Let’s Make Things Right
If you have used or currently use Exactech Optetrak, Optetrak Logic, Truliant, or Vantage systems and either had to undergo revision surgery or have suffered from osteolysis (bone loss), loosening, lysis, swelling, difficulty walking, and pain associated with polyethylene wear, you may be entitled to compensation.
Our team at Oppenheim Law recognizes the burden these complications and avoidable surgeries can have on both individuals and their lives. That is why our firm provides a team of professionals committed to zealously represent our clients.
Please feel free to call us at (954) 384-6114, contact us at contactus@oppenheimlaw.com, or chat with us at www.oppenheimlaw.com so we can inform you of your legal rights so you can obtain the compensation to which you are entitled.